3. Membrane for fuel cells (Operating Mechanism and Technological Issues)
With the marketing of fuel cell vehicles, it has become possible to analyze practical real-world performance advancement.
However, in order to promote advances in development, there are increasingly higher demands for improvement of analysis and simulation technologies of the basic performance and principles concerning power generation fuel cell, which is a core of the technology.
For this development, the capabilities and issues related to what kind of performance can be realized through the reaction mechanism at the molecular level in the cell of the fuel cell, as well as the movement of matter will be explained.
1.Construction of the Cell Reaction Area
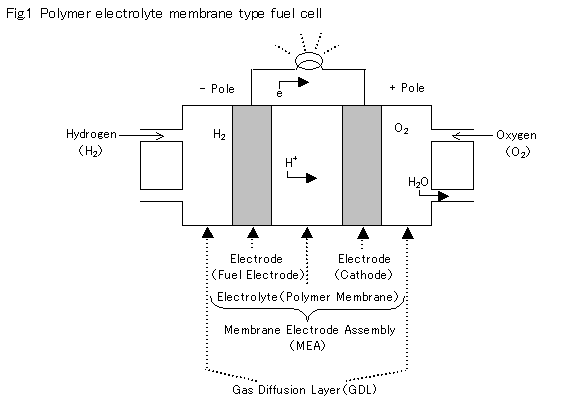
2.Gas Diffusion Layer (GDL)
The gas diffusion layer controls and supplies reaction gasses (hydrogen and oxygen) to the catalyst on the electrode while controlling the gasses. Currently, each layer is made of carbon fiber in a paper form. The movement speed@@the molecular of gasses depends of its weight.
Diffusion is very difficult on the air pole side (cathode side) where water generates when compared to the hydrogen pole side where molecular movement speed is higher. If the output varies, the amount of water generated fluctuates accordingly.
However, if drainage is improved the electrode dries out in local areas, and does not perform well. Furthermore, / if the part is
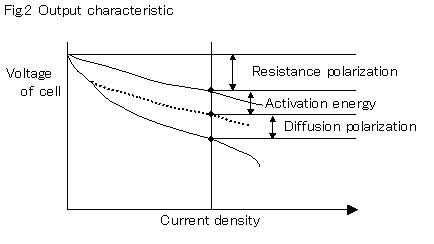
allowed to retain moisture, water condensation accumulates, which prevents air from reaching the electrode. Because the rate controlling of the output currentAis performed by diffusion polarization according to this design, draining performance is maintained within the normal use operation range by some designs such as an improvement in fine form, heterogeneous processing of hydrophilic or water repellent properties, and humidifying gasses. However, as the surface area of the membrane increases, partial drying or condensation in areas cannot be avoided. Currently, if the condensation side is used, the water diffusion is inadequate, polarization occurs, and the output threshold is reached.
3.Membrane Electrode Assembly (MEA)
A membrane electrode assembly is made by allowing a membrane electrode made of resin with an ion exchange capability to be put between the electrodes. However, only allowing the membrane electrode to be put between electrodes will result in ineffective functioning of the catalyst because there will be an insufficient area of electrolyte, resulting in hindering the flow of ions. Therefore, in case of a one-piece molding, MEA is made by adding mixed electrolyte particles to the electrode.
3-1 Fuel Electrode
The carbon particles that transmit electron are hardened and formed, and then covered with ultra fine platinum particles.
When hydrogen gas particles collide with and get absorbed by the platinum surface, the freedom of movement decreases. As a result, the particles are broken down to atoms. Furthermore, acceleration occurs along the surface, and some break away from the platinum surface in ionized form at the electrolyte interface. Due to inter-electrode potential, these hydrogen ions move within the electrolyte towards the air pole (cathode), which generates a current source. The free electrons emitted during ionization flow through the carbon electrode, carrying an electrical load.
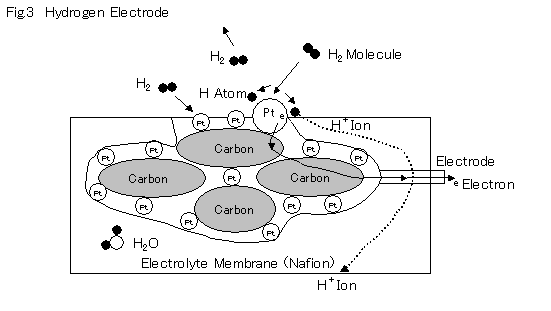
As catalysis is done by the platinum surface, fine particles of platinum (platinum black) are used to increase the surface area.
However, the issue at hand is how to make the particles even finer to achieve a platinum usage of one tenth of the status quo. Currently, over 100g of platinum is used for a single FCV.
With finer particles a higher state of surface energy is maintained. Accordingly, technical issues such as maintaining the stability (breakaway, particle gathering) of the electrolyte on the carbon become significant.
3-2 Cathode
The platinum electrode for the air electrode is common with the fuel electrode. For the air electrode, water is generated by the reaction between the hydrogen ions carried through the electrolyte and the oxygen absorbed by the platinum surface.
The chemical reaction between hydrogen and oxygen results in the conversion into electric energy because the hydrogen ion receives an electron. What differs significantly from the fuel pole is that there is plenty of water generated on the platinum surface. Oxygen that is absorbed is combined with the hydrogen ion sub sequentially to turn into a form of HOOH.
Then water is generated when exceeding a potential level of approx 0.5eV.
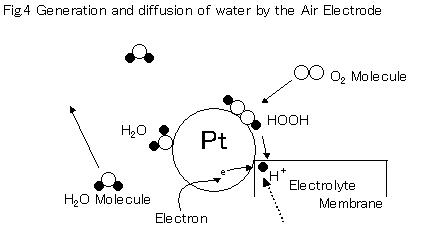
A higher potential (approx. 0.5eV)is required for water to then separate from the platinum surface.
Accordingly, removing water on the surface will significantly facilitate reaction speed. Additionally, HOOH causes separation of carbon of electrolyte membrane, which could result in a failure due to deterioration if active oxygen is generated (depends on the conditions in which water is generated). Therefore, a design of a catalyst in which separation of water is facilitated while suppressing the generation of active oxygen is needed. However, it is difficult to control the reaction if operating temperatures and output fluctuate in a short time.
3-3 Electrolyte Membrane
Currently, a perfluoro type ion exchange resin (Nafion) is a major electrolyte. With a low heat resistance of 80C, reliability and durability have become issues. From a cost point of view it is still expensive (100,000 yen/ m2).
The functional characteristics required by membranes are, gas sealing properties, ion transferability, and electrode insulation properties.
With regards to gas sealing, if the hydrogen penetrates the membrane and goes directly to the air electrode side, it is combined with the oxygen above the catalyst. However, as there is no electrical effect, no electricity is generated, and it is useless as a fuel. As the permeating capability of hydrogen molecules is high, a long time sealing capability is insufficient.
An MEA construction design that allows many reactions to occur at the hydrogen electrode is required.
A thinner membrane provides better reaction performance.
However, there are limitations with regards to strength against hole opening. A study to increase the strength of the membrane by providing a reinforcing net is underway.
The ion exchanging resin membrane function is the mechanism for transferring hydrogen ions.
The elements that perform the ion exchange are water and a sulphone group. Transferring driving forces are electric field and molecular motion.
First, the hydrogen ions separated at the hydrogen electrode become hydrated, and form H+ 3(H2O) .The mass that has a plus charge gets pulled towards the air pole by the electrical field. It's like sending funds by putting money directly into a registered cash delivery. This is referred to as "Vehicle".
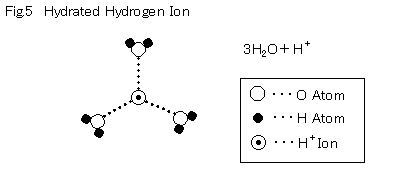
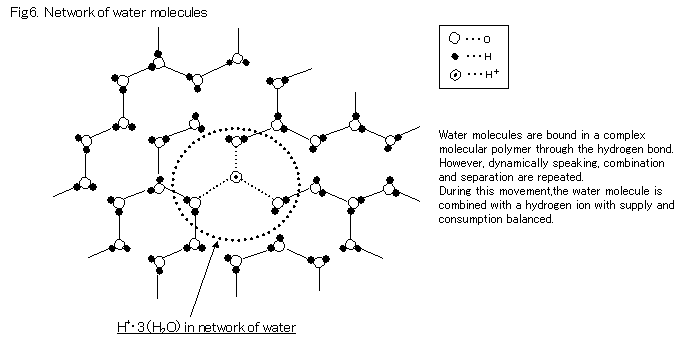
Another type of movement is so-called g Grotthus proton translocation g that can be likened to a monetary wire transfer from bank and bank. There are 2 types. One type of network Grotthus occurs when H+ enters a network of water molecules.
Through a change in the network construction as a result of molecular motion, the H+ is diffused, and statistically offsets the H+ consumed by the air electrode.
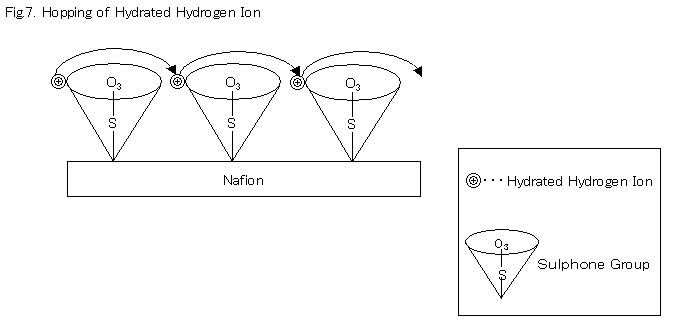
Another type is a movement referred to as "hopping".
This occurs when hydrated ions are transferred like stepping stones through a rotating motion of the oxygen portion of the sulphone group at the Nafionfs end.
However, because it is difficult to measure with molecules in motion, the rate of contribution for transferability of an ion in this mechanism is not clearly understood. The ratio of these mechanisms that contribute to proton transfer is not clearly understood.
Therefore, the basic performance depends significantly by reaction control according to material design, temperature, humidity and pressure at a molecular level. In order to resolve issues such as performance improvement and maintaining of sufficient durability, a leading edge research in fundamental technologies such as measurement technology and simulation methods will play a vital role in the design of materials where analysis is conducted at the molecular movement level.
|